Mihriban Karaayvaz Laboratory
-
Mihriban Karaayvaz Laboratory
- Principal Investigator
- Research
- Our Team
- Publications
- Careers
- Research News

Mihriban Karaayvaz, PhD
Assistant Staff
Email: [email protected]
Location: Cleveland Clinic Main Campus
Research
The Karaayvaz Lab focuses on understanding the early stages in the development of breast cancer in high-risk patients and how we may use that information to build novel cancer preventive measures.
The main area of interest in Karaayvaz lab is to better understand the mechanisms of breast cancer initiation in germline BRCA1/2 mutation carriers, a significant group of high-risk patients. Our research begins with the analysis of patient samples, followed by the development of hypotheses based on our observations and the building of experimental models to test these hypotheses in order to translate our findings into clinical care.
Biography
Dr. Karaayvaz is an Assistant Professor of Molecular Medicine at Lerner College of Medicine of Case Western Reserve University (CWRU) and an Assistant Staff in the Genomic Medicine Institute of the Cleveland Clinic Lerner Research Institute. She obtained her B.S. form Bilkent University; her Ph.D. degree from Stony Brook University, where she received an outstanding young investigator award; and completed her postdoctoral training at the Massachusetts General Hospital Cancer Center.
After she was recruited to the Cleveland Clinic in 2021, Dr. Karaayvaz received an American Cancer Society Institutional Research Grant to study the link between BRCA2 haploinsufficiency and common fragile site instability in human mammary epithelial cells. Her lab currently receives in-kind support from the Cleveland Clinic - IBM Discovery Accelerator program to apply cutting-edge machine learning algorithms to omics- and imaging datasets to improve breast cancer risk assessment in high-risk patients.
Education & Professional Highlights
EducationUndergraduate Student– Bilkent University
Molecular Biology and Genetics
Ankara, Turkey
2008
Graduate Student – Stony Brook University
Molecular and Cellular Biology
Stony Brook, NY USA
2013
Postdoctoral Fellowship – Massachusetts General Hospital
Breast Cancer Biology
Boston, MA USA
2019
- Instructor, Massachusetts General Hospital Cancer Center and Harvard Medical School, 2019
- Assistant Staff, Genomic Medicine Institute of Lerner Research Institute at Cleveland Clinic, 2021
- Assistant Professor, Molecular Medicine Lerner College of Medicine of Case Western Reserve University, 2022
- Outstanding Young Investigator Award, Stony Brook University, 2013
- Awarded by Susan G. Komen Postdoctoral Fellowship Grant, 2016
- Awarded by Terri Brodeur Breast Cancer Foundation Postdoctoral Fellowship, 2016
Research
Overview
Our laboratory focuses on understanding the early events in breast cancer development. Our research begins in the clinic, with the analysis of patient samples.We develop hypotheses based on our observations, and build experimental models to test these hypotheses with the goal of translating our findings into clinical care.Two of our main research focuses can be found below.
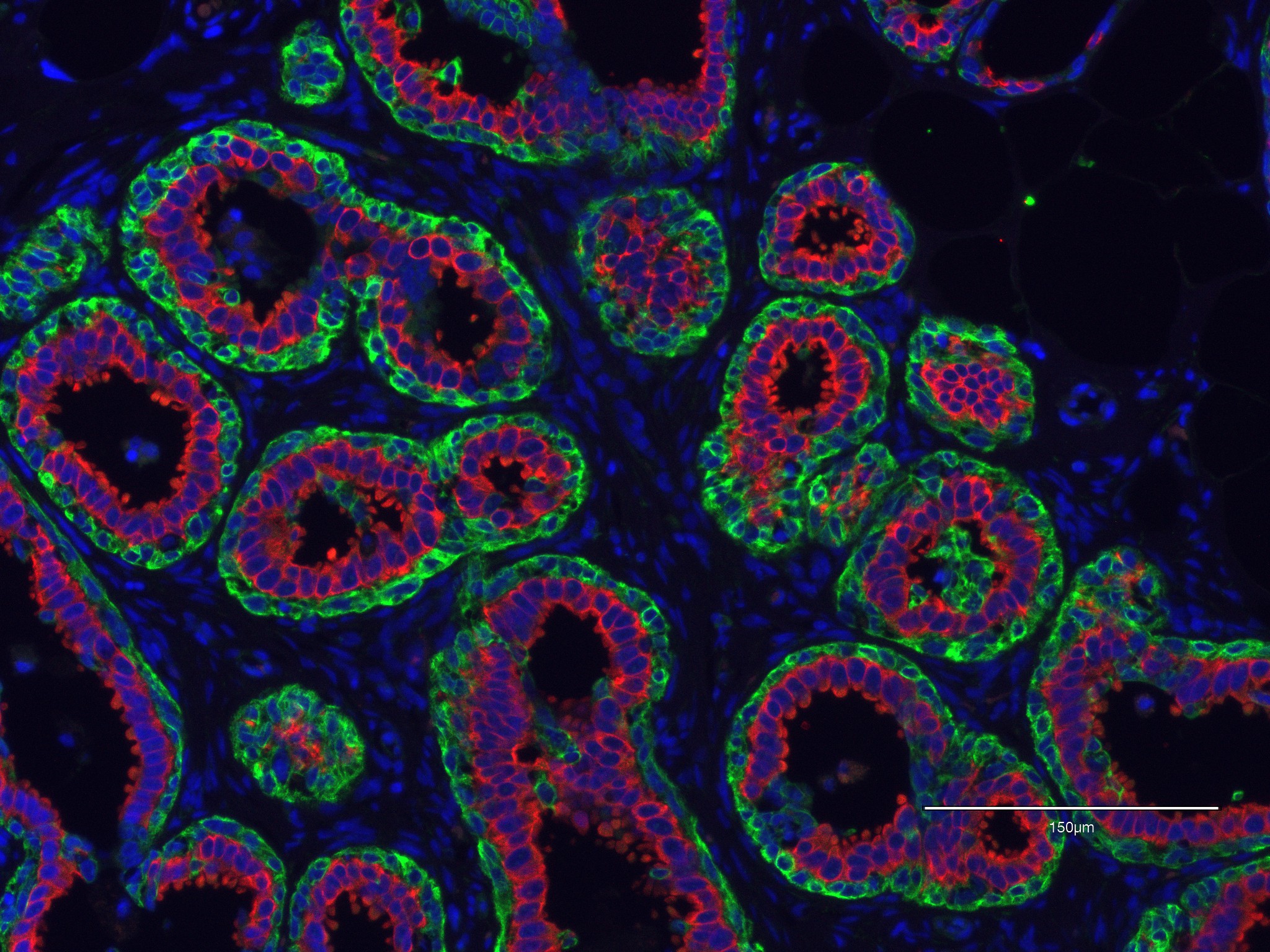
Consequences of BRCA1/2 haploinsufficiency
Individuals with germline heterozygous mutations in the breast cancer susceptibility genes BRCA1 or BRCA2 have a 70% lifetime risk of developing breast cancer, yet the mechanisms of these cancers remain largely unknown. There is growing evidence that BRCA1 and BRCA2 heterozygosity confers haploinsufficiency in normal human mammary epithelial cells for their multiple known functions. A major focus of the Karaayvaz lab is to determine the cellular effects of this BRCA1/2 haploinsufficiency in human breast epithelial cells.
BRCA1 haploinsufficiency leads to defects in normal mammary epithelial progenitor differentiation and to replication stress suppression in primary cells. Haploinsufficiency for BRCA1 also causes cell-type specific genomic instability and premature senescence.
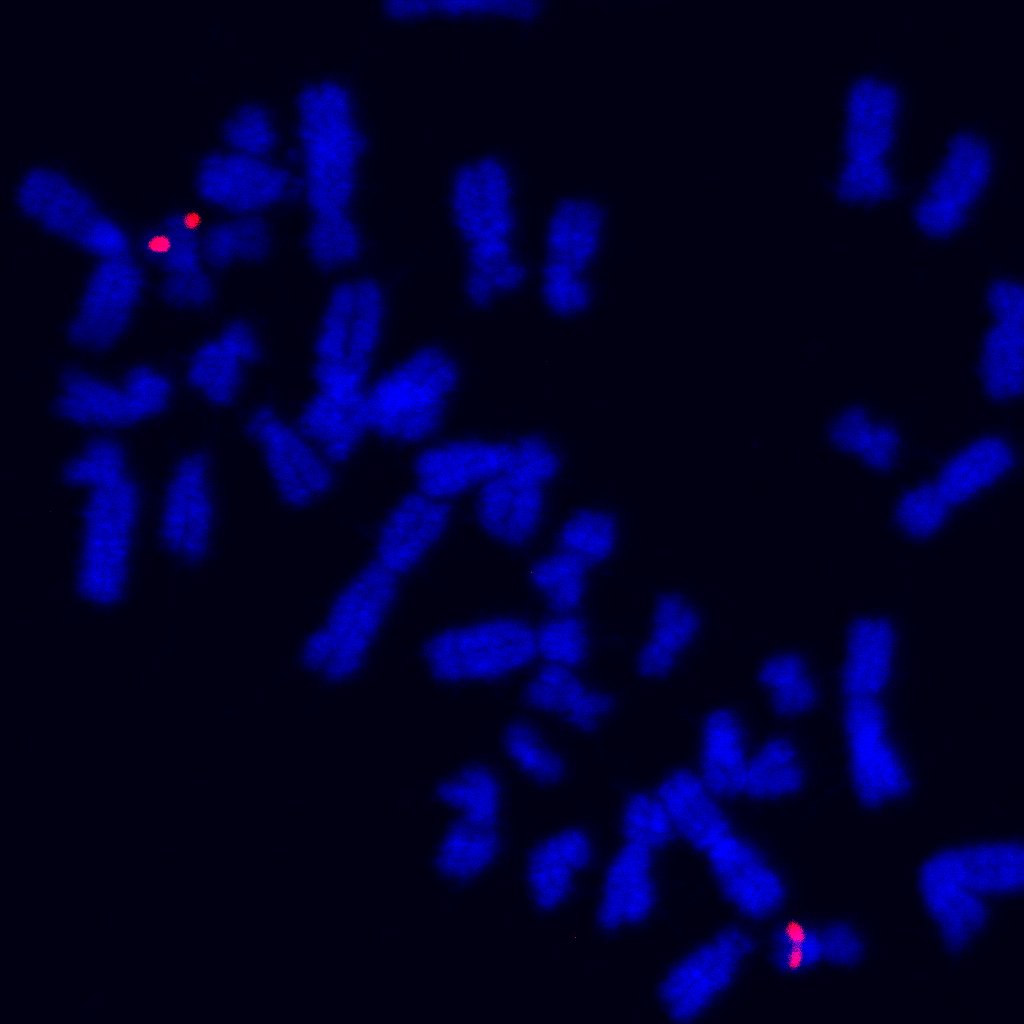
There is relatively less research on understanding the role of haploinsufficiency for BRCA2 in normal human mammary epithelial cells. It has been reported that haploinsufficiency for BRCA2 leads to genomic instability via unscheduled R-loops. Our work demonstrated that BRCA2 haploinsufficiency and associated DNA damage precede histologic abnormalities in BRCA2 mutation carriers. We discovered that single cells isolated from normal breast tissues of patients who carry germline truncating mutations affecting BRCA2, harbor frequent sub-chromosomal copy number variations (CNVs) (Science Advances, 2020). Correspondingly, these cells also exhibit attenuated DNA damage and apoptotic responses.
Role of microenvironment in cancer-predisposed human breast tissues
Defects in mammary epithelial cell differentiation are present in BRCA1/2 mutation carriers even before cancer incidence. However, the underlying mechanism is largely unknown. Currently, we are investigating the influence of discovered receptor-ligand interactions on mammary epithelial cell differentiation using patient-derived experimental models that we are building in our lab.
Cell fate decisions are driven by changes in environmental signals. Thus, we performed spatial transcriptomics using breast tissues from BRCA1/2 mutation carriers and from non-mutation carrier controls to study defects in mammary epithelial cell differentiation accompanied by unique microenvironmental alterations. We uncovered spatially defined receptor-ligand interactions in these tissues for the investigation of autocrine and paracrine signaling. We revealed both differences and similarities in the communication between mammary epithelial cells and the microenvironment in BRCA1 and BRCA2 mutation carriers (bioRxiv, 2023).
Our Team
Selected Publications
View publications for Mihriban Karaayvaz, PhD
(Disclaimer: This search is powered by PubMed, a service of the U.S. National Library of Medicine. PubMed is a third-party website with no affiliation with Cleveland Clinic.)
Careers
We are always looking for dedicated and enthusiastic individuals to join our team!
Training at Lerner Research Institute
Our education and training programs offer hands-on experience at one of the nationʼs top hospitals. Travel, publish in high impact journals and collaborate with investigators to solve real-world biomedical research questions.
Learn MoreResearch News

The study used breast tissue samples from Cleveland Clinic’s biorepository to shed light on breast cancer’s biological beginnings.

With a grant from the American Cancer Society, Dr. Karaayvaz’s team will investigate the molecular connections linking BRCA2 to breast cancer development with the goal of developing innovative strategies for breast cancer risk assessment and prevention.