Bin Zhang Laboratory
-
Bin Zhang Laboratory
- Principal Investigator
- Research
- Our Team
- Publications
- Careers
- Research News
Research
All proteins in our bodies are made within cells to perform specific tasks, but not all proteins start out where they’re needed. Many must travel to their job sites in different parts of the cell, or even to locations outside their parent cell. Up to one-third of these proteins must traverse a tightly regulated pathway called the secretory system in their journeys. The Zhang laboratory is primarily interested in learning the details of how the protein secretory pathway works and how deficiencies in this pathway ultimately relate to human diseases.
One of the most famous examples of a secretion pathway deficiency disease is hemophilia, in which pathway deficiencies create roadblocks that block blood clotting factors in the liver from entering the bloodstream. We primarily study how secretory pathway deficiencies cause hemophilia and other bleeding disorders, but we are beginning to investigate other diseases such as anemia and cancer. Our lab uses biochemistry, cell biology, molecular genetics, and in vivo preclinical models to study how these diseases happen by determining how the secretory pathway functions on its most basic level, step-by-step. We have also recently begun to computationally analyze publicly available patient genome data to uncover the genetic basis of these diseases.
After all, to fix a broken car you need to know how it works and you need to know where to look!
Biography
Dr. Bin Zhang is an Associate Staff member in the Cleveland Clinic’s Department of Genomic Medicine, and an Associate Professor at the Lerner Research Institute College of Medicine.
He is interested in the regulation of protein secretion, with a major focus on understanding the mechanism of factor V, factor VIII, and α1-antitrypsin transport from the ER to the Golgi body. As a PI of several NIH- and Foundation funded grants, he has led his research team to achieve significant progress in understanding the mechanism of the receptor-mediated secretion of FV and FVIII, the pathogenic mechanisms of missense and nonsense mutations in hemophilia A and the in vivo functions of coat protein complex II components in the mammalian system. In recent years, his laboratory has turned to use their knowledge of FVIII translational and posttranslational regulation to develop novel therapeutics to treat hemophilia A. Previous work also allowed his group to discover new ways to bioengineer the FVIII molecule to improve its secretion and apply novel FVIII variants to hemophilia A gene therapy applications.
Education & Professional Highlights
Education
B.S. - Biochemistry, Wuhan University
M.S. - Biochemistry, Shanghai Institute of Biochemistry, Academia Sinica
Ph.D. - Biochemistry, Michigan State University
Postdoc – Biochemistry, Michigan State University
Postdoc – Human Genetics, University of Michigan
Professional Memberships
2011- Member, International Society of Thrombosis and Haemostasis
2015- Member, American Society of Hematology
2021- Editorial board member, PloS One
Research
Overview
We are particularly interested in the components of the secretory pathway that assist secretory proteins in the first step of leaving their original cell. To leave the cell, these proteins must first move from the endoplasmic reticulum (ER), where they are made, to the Golgi apparatus (Golgi). Our main areas of focus are the processes that allow secretory proteins travel between these cellular compartments within vesicles. See some of our ongoing projects below!
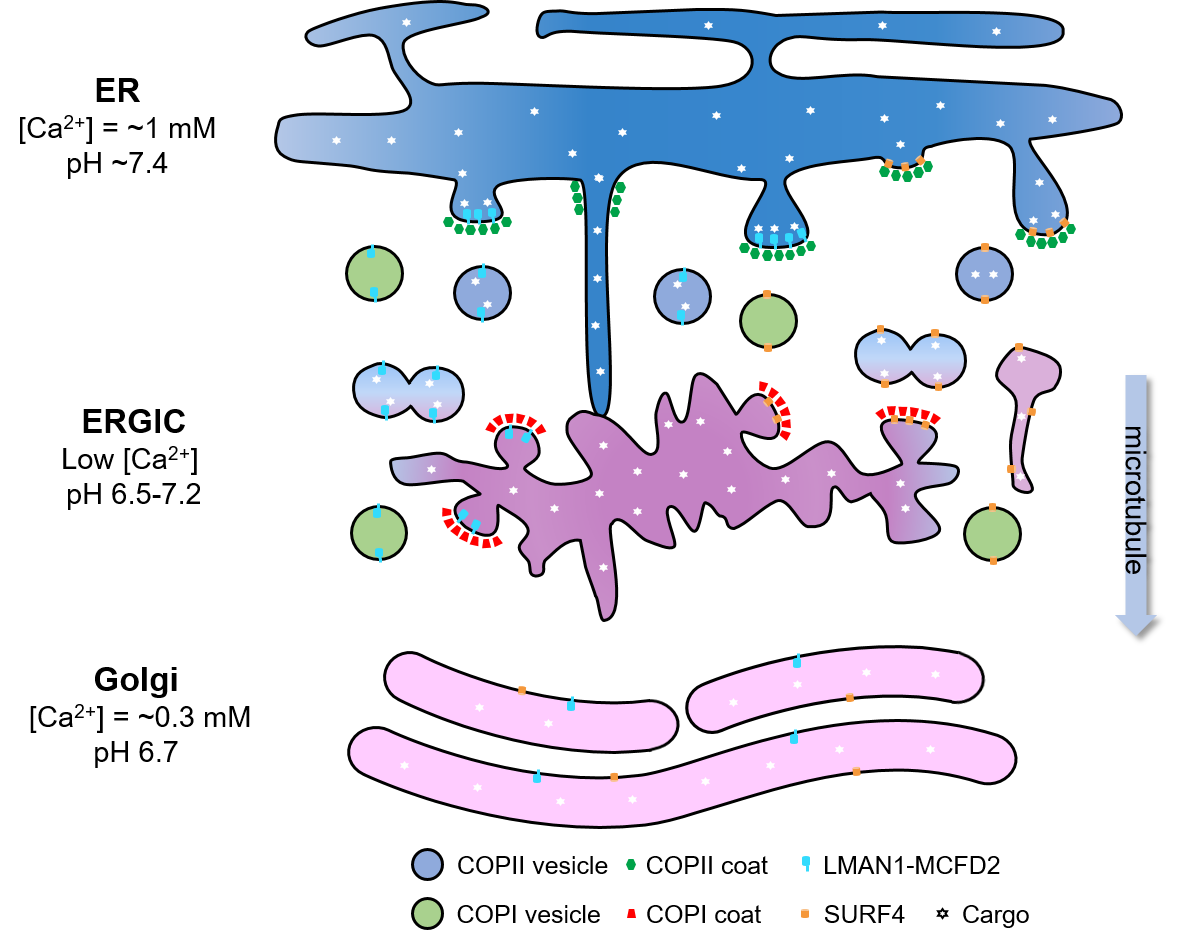
pictured: the early secretory pathway
Cargo Receptors and their impact on bleeding disorders
Knowing which secretory proteins to transport from the ER to the Golgi is a tightly regulated process. Cells use molecules called cargo receptors that bind to specific proteins during vesicle formation. This binding process prevents other proteins and low-quality secretory proteins from entering the vesicle and acting as stowaways from the ER to the Golgi.
The best-known mammalian cargo receptor is the LMAN1-MCFD2 protein complex. Mutations in either gene can lead to a bleeding disorder called combined deficiency of factor V and factor VIII.
Using biochemistry, cell biology, molecular genetics, and preclinical in vivo models, researchers in the Zhang lab characterize the organization of the LMAN1-MCFD2 complex to elucidate how it recognizes and binds properly folded proteins in the ER and selectively releases its cargo in the post-ER compartments. The knowledge gained from these studies can help us improve factor VIII secretion in vivo and in vitro that will benefit patients with hemophilia A.
Recent evidence suggests additional functions of this cargo receptor complex beyond the transport of clotting factors. We are thus seeking to understand its role in other biological processes, such as cancer development, and to identify additional proteins that are secreted through this pathway.
Vesicle formation, COPII, and human disease
Proteins that exit the ER are packaged into COPII-coated vesicles. Mammalian cells contain multiple isoforms of the COPII proteins. Recent genetic studies have attributed distinct human disorders, including pancreatitis (inflammation of the pancreas) and Cowden Syndrome (tumor overgrowth), to mutations in specific COPII isoforms. We use preclinical in vivo models to study the mechanisms of inherited diseases caused by deficiencies of individual COPII isoforms and gain insight into the in vivo functions of these important proteins.
Engineering Gene Therapies
Through our research, we have a sophisticated understanding of how factor VIII secretion works – and fails to work – in human health and disease. Our current goal is to develop gene therapies to restore an individuals’ secretory pathways and potentially mitigate or even reverse their bleeding disorders. However, developing gene therapy for these types of genes presents unique challenges. We are currently working to overcome the technical hurdles involved in developing these therapies including:
- Determining how best to express the gene
- How to deliver the gene to the proper location
- What vector to use, and how much of the large factor VIII gene we need to insert in the vector
- How to maximize the therapeutic response to this therapy while minimizing potential side-effects and immune reactions
Our Team
Selected Publications
View publications for Bin Zhang, PhD
(Disclaimer: This search is powered by PubMed, a service of the U.S. National Library of Medicine. PubMed is a third-party website with no affiliation with Cleveland Clinic.)
Selected publications:
1. Zhang Y, Liu Z, Zhang B. Separate roles of LMAN1 and MCFD2 in ER-to-Golgi trafficking of FV and FVIII. Blood Adv. 2023 Apr 11;7(7):1286-1296. doi: 10.1182/bloodadvances.2022008788. PubMed PMID: 36490287; PubMed Central PMCID: PMC10119632.
2. Zhang Y, Zhu M, Zheng C, Wei W, Emmer BT, Zhang B. LMAN1-MCFD2 complex is a cargo receptor for the ER-Golgi transport of α1-antitrypsin. Biochem J. 479(7):839-855. 2022. doi: 10.1042/BCJ20220055. PMCID: PMC9022998
3. Wei W, Liu Z, Zhang C, Khoriaty R, Zhu M, Zhang B. A common human missense mutation of vesicle coat protein SEC23B leads to growth restriction and chronic pancreatitis in mice. J. Biol. Chem. 298(1):101536. 2022. doi: 10.1016/j.jbc.2021.101536. PMCID: PMC8760524
4. Liu Z, Zhang Y, Zhu M, Zhang B. Identification of candidate nonsense mutations of FVIII for ribosomal readthrough therapy. Haematologica. 104: e573-e576. 2019. PMCID: PMC6959177.
5. Zhu M, Zheng C, Wei W, Everett L, Ginsburg D, Zhang B. Analysis of MCFD2 and LMAN deficient mice demonstrate distinct functions in vivo. Blood Adv. 2(9):1014-1021. 2018. PMCID: PMC5942004.
Careers
The Zhang Lab promises to foster a positive laboratory environment of respect, excitement, innovation, and support. Science is a team sport, and our team is committed to supporting each other on their journeys to reach their full potential as researchers and individuals
Training at Lerner Research Institute
Our education and training programs offer hands-on experience at one of the nationʼs top hospitals. Travel, publish in high impact journals and collaborate with investigators to solve real-world biomedical research questions.
Learn MoreResearch News

The discovery provides answers to children and families affected by neurological symptoms with no known cause.

With their awards, Drs. Srivastava and Zhang will study the blood-clotting protein factor VIII.

The National Heart, Lung, and Blood Institute awarded Bin Zhang, PhD a four-year, $1.6 million grant renewal.
